Healthy, young volunteers who have previously had COVID-19 will be deliberately exposed to coronavirus for a second time to see how the immune system responds, as part of a new U.K. study.
Researchers at the University of Oxford on Monday launched the “human challenge” trial to investigate what happens when volunteers who have recovered from the coronavirus disease are then reinfected with the virus a second time.
The study, which is funded by the Wellcome Trust, is expected to start in the next few weeks after receiving ethics approval, and could help accelerate the development of new treatments and vaccines against the disease.
Human challenge studies have played a crucial role in the development of treatments for a number of diseases, including malaria, typhoid, cholera and flu.
Read: Only 50 people are known to have contracted COVID-19 more than once — but new strains have medical experts on high alert
“A challenge study allows us to make these measurements very precisely because we know exactly when someone is infected,” said Helen McShane, professor of vaccinology at the department of pediatrics at the University of Oxford and chief investigator on the study.
“The information from this work will allow us to design better vaccines and treatments, and also to understand if people are protected after having COVID, and for how long,” McShane said.
Read: COVID-19 infection likely to provide immunity for at least 5 months, but people may still transmit virus, study finds
The first stage of the trial will involve up to 64 volunteers aged 18-30 who have previously been naturally infected with COVID-19. It will look to establish the lowest dose of virus that can take hold and start replicating in about 50% of participants, while producing few to no symptoms.
Volunteers will be monitored in a safe, controlled environment while quarantined in a specially designed hospital suite for a minimum of 17 days. Anyone who develops coronavirus symptoms will be given Regeneron
REGN,
monoclonal antibody treatment.
Once the standard dose is established, it will be used to infect different volunteers in the second stage of the trial, which is due to start in the summer. The full length of the study will be 12 months, including a minimum of eight follow-up appointments after volunteers have been discharged.
Read: Young, healthy adults will be paid £4,500 to be deliberately infected with COVID-19 in new trial
The new study is different to a parallel one led by Imperial College London, which was announced in February, and will expose up to 90 carefully selected healthy adult volunteers to coronavirus to help researchers understand how the virus infects people and how it is transmitted.
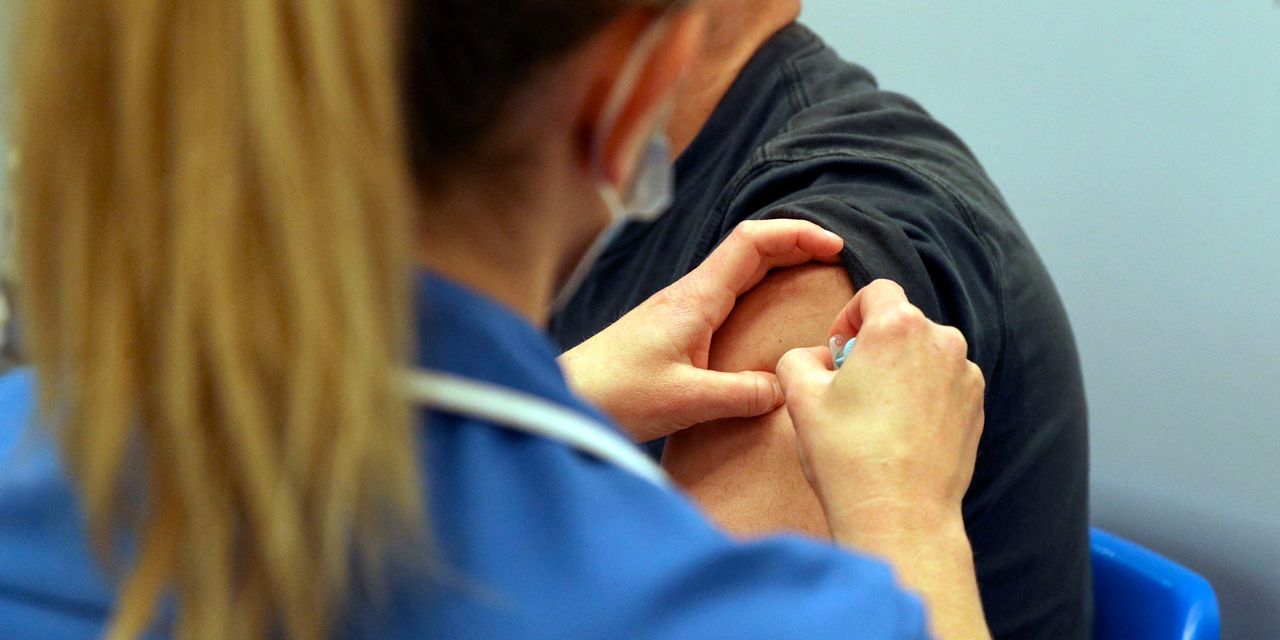
It comes as almost 10 million people in the U.K. have now received their second dose of the COVID-19 vaccine, according to the latest government figures.
Three vaccines are currently in use in the U.K.: the one jointly developed by German biotech BioNTech
BNTX,
and U.S. drug company Pfizer
PFE,
; the one produced by drug company AstraZeneca with the University of Oxford; and the shot from biotech Moderna
MRNA,
Last week, Moderna said it would deliver fewer than expected COVID-19 vaccines to the U.K., Canada and other countries, following a shortfall in production in its European supply chain.