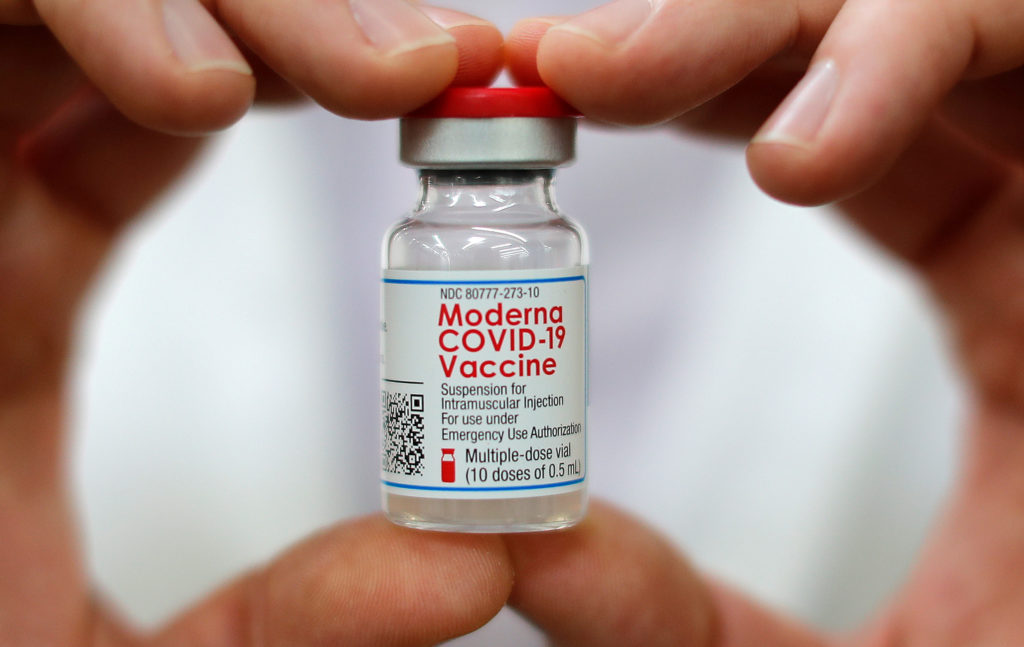
Two of the three COVID-19 vaccines that have been authorized so far in the United States use synthetic messenger RNA, or mRNA, to protect against the coronavirus. Though these vaccines — developed by Pfizer and Moderna, respectively — are the first of their kind to be used at this scale, this historic moment would not be possible without the decades of research that came before it.
There are lots of different ways to make a vaccine, but the ultimate goal of any shot is to introduce the body to the biological equivalent of a “most wanted” poster so that if the real enemy ever shows up, our immune systems know how to fight it off.
For some vaccines, that poster is a version of a pathogen that’s been weakened — like the chickenpox shot — or inactivated — like most flu shots — so that it can’t actually cause infection. For others, including the HPV and shingles vaccines, it’s a piece of that pathogen, like the specific protein it uses to infect cells in the first place.
But mRNA vaccines take a different approach. Rather than tinkering with the virus or its parts, this platform harnesses the “beauty of our biology” to deliver protection, said RNA virologist Paul Duprex, who directs the University of Pittsburgh Center for Vaccine Research. These vaccines teach the body to remember one of the coronavirus’ defining features — its spike protein — and prompt the creation of antibodies that can prevent it from infecting cells.
Our DNA resides inside the nucleus of our cells. Every day, mRNA molecules constantly carry genetic information coded in that DNA from the nucleus to the parts of the cells, called ribosomes, that can interpret those messages and then make the proteins that carry out essential biological processes. Without it, life would be impossible.
“Pretty much every single cell in my body at this particular moment is producing billions and billions and billions of messenger RNAs,” Duprex said.
READ MORE: The essential COVID-19 vaccine FAQ
Vaccines that use synthetic mRNA add one more type of mRNA to the legion of other molecules “doing their daily business” within our bodies, and use it “to make a protein which the immune system will see and make antibodies against and protect us from a disease,” Duprex added.
Around 20 years ago, the work of two researchers — Drew Weissman and Katalin Karikó — helped overcome two primary barriers that had been standing in the way of utilizing mRNA technology: an inflammatory effect on the body that made test animals ill, and the fragile nature of the molecule itself, both of which hindered its utility.
Despite those advancements, and the wealth of research that’s been carried out since, the fact remains that the two mRNA vaccines in use today are the first of their kind. That may be in part because it’s difficult to generate interest and funding to support pursuing “non-mainstream” science outside of a crisis, Duprex said — what he characterized as “a shortsighted way to think about biology.
Only now, amid a devastating pandemic, has this technology reached mainstream prominence. “Given the choice, I would have rather avoided this past year,” Weissman said. “But we didn’t, and now RNA is going to be our future.”
Here’s a look at how, exactly, these vaccines manage to pull off this feat and some of the key research breakthroughs that made this moment possible.
How messenger RNA vaccines work
In order to develop these vaccines, researchers took the RNA-based genetic sequence of the coronavirus and turned it into DNA. This crucial step allowed them to identify the “instructions” necessary to create the spike protein, engineer corresponding synthetic mRNA and package that into their vaccines.
mRNA, as its moniker implies, is a messenger. This particular type of RNA is tasked with delivering messages to microscopic cellular machines called ribosomes, located in the cytoplasm of our cells, which are responsible for synthesizing proteins. Those ribosomes then interpret that message to make proteins and start executing its instructions, explained Phillip Sharp, a molecular biologist and MIT professor who shared the 1993 Nobel Prize in physiology or medicine for his contribution to our understanding of RNA.
Dendritic cells, the watchdogs of the immune system, play an essential role in responding to pathogens. They patrol the body in search of foreign invaders and, when they find one, start stimulating an immune response. When these cells encounter mRNA that’s been injected via vaccination, their ribosomes decode the message and allow the cells to temporarily display spike proteins identical to the ones found on the coronavirus’s exterior, Weissman said.
“Dendritic cells make the spike protein and then they present it to other immune cells and activate them to start the immune response,” he added.
The proteins allow the dendritic cells to alert two more key players in the immune system — T cells and B cells — that if they see those same spikes on any other cell, they should recognize them as a foreign invaders and either destroy them or generate antibodies to neutralize them immediately.
“There’s a memory component of those cell populations, and that stays in your body over a long period of time,” Sharp said. “If a similar virus infects you, those memory cells are ready to go. They are all perfected to go out and kill that virus.”
mRNA naturally degrades rapidly over time, so once it has served its purpose, it simply breaks down. The dendritic cells that expressed the spike protein eventually die and are replaced by new ones that continue to pick up that vaccine-delivered mRNA and repeat the process all over again in the course of about two weeks following immunization.
Some members of the public have expressed concern over unfounded speculation that these vaccines could negatively affect the body. But it is impossible for an mRNA vaccine to alter your DNA because synthetic mRNA operates only in the cytoplasm and is incapable of entering any other parts of our cells, such as the nucleus.
Like virtually all vaccines, those that use mRNA can trigger temporary symptoms like a fever, fatigue and soreness at the injection site that dissipate within a few days. But clinical trials that took place before the vaccines were authorized, as well as those that have followed, all suggest that these vaccines are both safe and effective at preventing serious illness and death.
“It’s always, always much more risky to get the disease than it is to get the vaccine,” Duprex said.
How did we get here?
mRNA was first injected into the muscles of mice in 1990 with the intention to deliver therapeutic proteins. But that effort “didn’t go very far,” according to Weissman, in large part due to the strong inflammatory response it induced, which severely sickened the animals involved.
That’s because in both animals and humans, cells feature a number of different receptors that can recognize mRNA as a foreign substance that must be destroyed. Those receptors help these cells distinguish their fellow cells from invaders like viruses, bacteria or even tumor cells.
Both RNA and DNA are composed of four nucleotides. More than a decade after that first injection in mice, Weissman and Karikó, who now serves as senior vice president at BioNTech, which partnered with Pfizer to manufacture their joint vaccine, figured out a way to insert an modified nucleotide that allows the synthetic mRNA to masquerade as a normal cell and circumvent those receptors, no longer triggering extreme inflammation. It also made the mRNA-spurred protein production more efficient.
“Our big discovery was that we could modify the RNA to make it non-inflammatory. And that had a couple of important features to it, but the first was that it greatly increased the amount of protein made off of the RNA,” which increased potency, Weissman said.
With the inflammation problem solved, Weissman and Karikó then turned to tweaking how mRNA is delivered so it could actually do its job once injected into the body. mRNA is an inherently “labile,” or unstable, material that can degrade rapidly to the point of being rendered ineffective.
After testing around 40 different types of delivery systems, the researchers found their golden ticket: lipid nanoparticles. These “droplets of fat” coat the mRNA and allow it to successfully enter our cells, which are also encapsulated in an oily substance.
Traditional vaccines are typically formulated with adjuvants that are designed to stimulate the immune response in their recipients. In what Weissman described as a lucky development, lipid nanoparticles happened to act as an adjuvant that stimulated a specific type of “helper cell” that promotes antibody responses.
“We use the lipid nanoparticles to get over a lot of the fragility [problems] because that protected the [mRNA] after you injected it into people, and it promoted these cells to take up the [mRNA] and start the vaccine process,” Weissman said.
Where mRNA stands today
In the years since Weissman and Karikó made these breakthroughs, mRNA research has continued to march on. Weissman and his current colleagues have worked on a variety of mRNA vaccines, including a “universal” flu shot that could cover a majority of influenza viruses and has so far proven to be effective in animal trials.
Compared to traditional vaccine platforms that require a series of complex steps, like growing mammalian cells in massive quantities and a viral purification process that looks different depending on the pathogen you’re working with, mRNA is now easy to manufacture at a fairly large scale.
Instead of needing “to reinvent the wheel every time you make a new vaccine,” Weissman said, “with [mRNA,] it’s the same reaction, and the only thing you have to do is plug in the new sequence for any virus, so that makes it very easy to produce a new vaccine.”
Both Moderna and Pfizer’s vaccines generated above 90 percent protection after two doses during clinical trials that played out before new variants of the virus marginally reduced their efficacy. Even so, the two give recipients remarkably high levels of protection, particularly against severe disease and death.
The CDC recently released new research that found these vaccines reduce a fully vaccinated person’s chance of getting infected with the coronavirus by 90 percent in “real-world” settings like the workplace.
Given that no vaccines have ever been approved to immunize people against any kind of coronavirus, and that the FDA’s original hope was to secure one with at least 50 percent efficacy to curb the pandemic, these results represent yet another significant milestone in annals of RNA technology.
Much more research lies ahead for these vaccines, both of which have been rolled out in the United States and in some other countries over the past few months. In addition to continuing to track safety and efficacy data, researchers need to know how well these vaccines prevent recipients from transmitting COVID-19 and how long the protection they offer lasts. Until we know the answers to those questions, recipients should keep following pandemic precautions like wearing a mask, even after they’ve gotten their two doses, experts say.
READ MORE: How to stay safe from COVID this summer, according to experts
Johnson & Johnson’s vaccine, a one dose shot that uses a different yet similarly innovative platform to deliver immunity compared to mRNA, has also been authorized for use in the United States. Its strong efficacy and ability to be stored at a less strict temperature range makes experts hopeful that the rollout of this vaccine will help close some gaps in vaccine access both in this country and abroad.
In tackling COVID-19, Pfizer and Moderna’s vaccines have “paved the way,” Duprex said, when it comes to illustrating the utility of synthetic mRNA. And yet, while he anticipates that researchers will “only get better” at making tweaks that allow for better delivery and stability of this technology, he notes that we’re still in the early days of harnessing its utility — we also can’t assume that mRNA is “the next big panacea” that will solve all of our problems.
But, Duprex said, “the beautiful thing about this is this just gives us another brush for the palette of novel therapeutics [and] novel ideas that somebody in the next generation of scientists are going to be able to [use to] paint.”