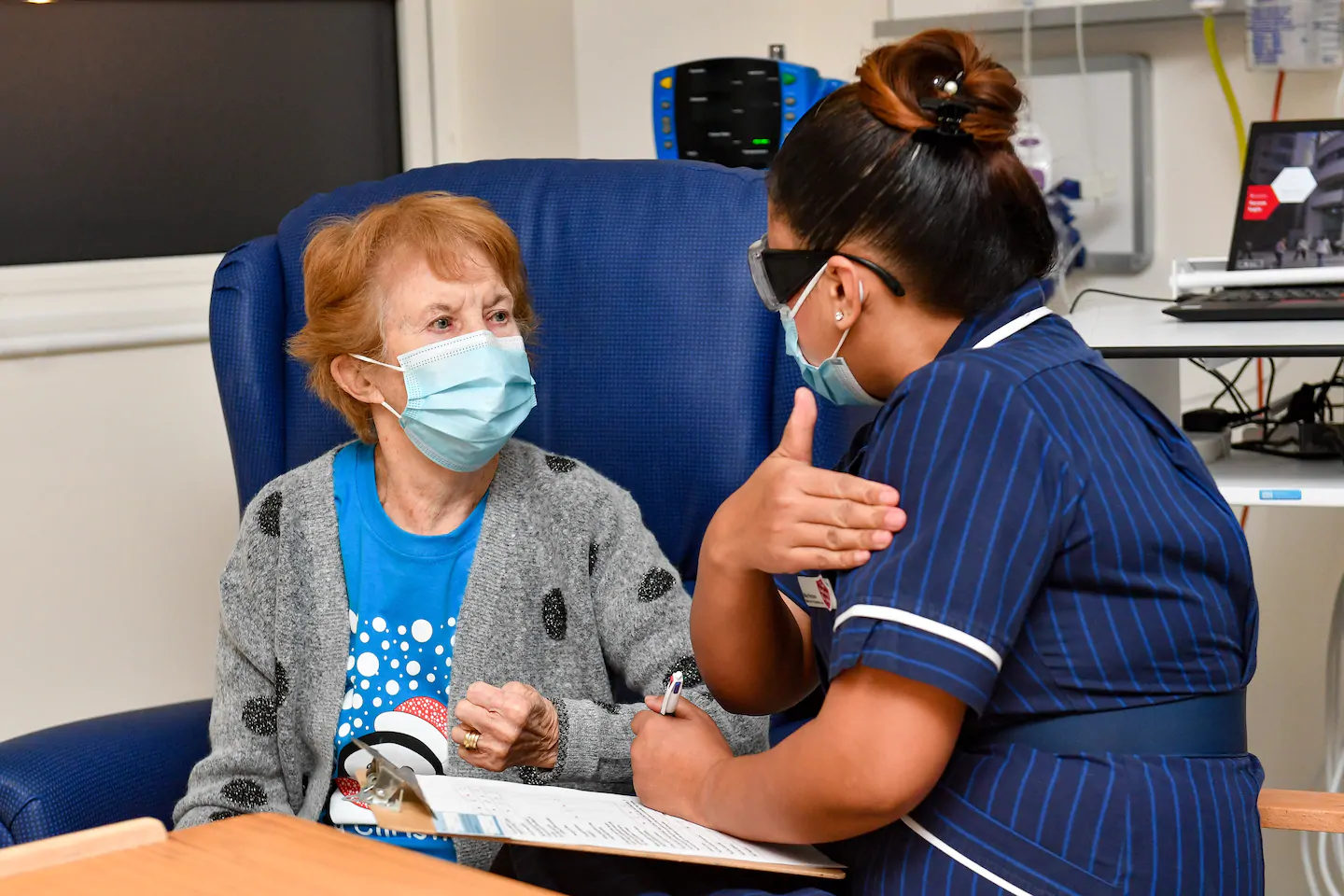
“I feel so privileged to be the first,” Keenan said, adding that it meant she could “finally look forward to spending time with my family and friends in the new year after being on my own for most of the year.”
That was one quick shot for Margaret, one giant leap for humankind.
“My advice to anyone offered the vaccine is to take it — if I can have it at 90 then you can have it too,” she said.
British health officials hailed the first injections as a turning point in the fight against a virus that has infected 67 million people around the globe, killing more than 1.54 million.
“I’m feeling quite emotional, actually, watching those pictures,” British Health Secretary Matt Hancock told Sky News, which broadcast footage of Keenan getting the jab live. “It’s been such a tough year for so many people and finally we have our way through it, our light at the end of the tunnel.”
And certainly the world is watching to see how the country where vaccines were invented three centuries ago rolls out its largest and speediest ever health campaign, deploying a revolutionary new vaccine that requires extremely special care and handling.
Thousands of nurses, pharmacists and medical technicians, bolstered by legions of volunteers and members of the British military, will safeguard, transport, unpack and jab millions of doses into the upper arms of a nation grown weary of lockdowns and loneliness, anxiety and sickness.
Very soon, other nations are expected to follow. The United States could grant emergency authorization to the Pfizer vaccine by the end of the week. Europe, by the end of the month.
The ultimate goal is to inoculate enough people to confer individual immunity and eventually stop the virus’s spread. But until more vaccine doses are available and other vaccines are approved, officials here and elsewhere are balancing the need to protect the most vulnerable against the need to slow transmission.
British officials say they hope to vaccinate “the majority” of especially vulnerable people by the end of February. Priority, though, will go to people over 80 years-old and to nursing home care givers.
It will be a while before shots make it to nursing home residents themselves, as the government doesn’t yet have the ability or approval to offer Pfizer injections at those facilities.
Also excluded from the initial round will be most front line hospital workers. That runs counter to what the United States and most other countries are planning. Though Chris Hopson, chief executive of NHS Providers, said high-risk hospital staff in Britain will be offered “any leftover spare doses” at the end of each day.
Even for Britain’s highest priority groups, demand could quickly outstrip supply in the early months, public health officials warned.
The country has preordered 40 million doses of the Pfizer product, enough to immunize 20 million people, as two doses are required, three weeks apart. It has also hedged its bets and reserved another 300 million doses of five vaccines in development.
But the Pfizer vaccine is the only one approved so far, and the 800,000 doses Britain is set to receive “could be the only batch we receive for some time,” Hopson cautioned last week.
The government says more Pfizer vaccine is coming soon, but exactly how much and when depends on the company’s ability to manufacturing capability at its plant in Belgium.
The vaccine, developed by a husband-wife team at the small German company BioNTech, is not like a traditional shot that injects a crippled version of a virus. Instead, it uses a bit of messenger RNA, which encourage the body to produce antibodies to repel the spike protein on the surface of the coronavirus.
The vaccine has shown great promise, demonstrating 95 percent protection in large-scale human trials. It also presents a great challenge. It needs to be stored and shipped at seriously cold, sub-Antarctic temperatures of minus 167 degrees Fahrenheit.
British regulators, in granting emergency approval, said it cannot be moved more than four times and that the trays of 975 doses packed in dry ice cannot be split apart.
Hopson said the first trays are being delivered from the freezers at National Health Service warehouses to 50 hospitals in the United Kingdom.
NHS officials said primary care physicians are providing lists of people over 80 who are mobile enough to get to a hospital clinic, and hospital appointment bookers are calling those people for timed 15-minute slots. Some afternoon slots will be reserved for nursing home staffers, who are being contacted by their employers.
Vaccination will take place in separate, dedicated clinics at the hospitals, so the elderly and others who come in do not come in contact with patients who might be infected with the virus.
“The electronic prescribing system will cleverly automatically book the patient for their three-week-later second dose appointment,” Hopson said. “It will also, equally cleverly, automatically send a letter to the patient and their GP with details of that appointment and first dose.”
China is also now rolling out a campaign to inoculate more than a billion people, and claims it has already injected a million of its citizens with one of its five new experimental vaccines.
Russia began a mass program to inject doctors, teachers and social workers in hard-hit Moscow over the weekend. It is not known how effective the experimental Chinese or Russian vaccines are, because the makers there have reported scant data on clinical trials.
Britain was the first country in the West to approve a coronavirus vaccine.
In the United States, the Food and Drug Administration could grant emergency authorization to Pfizer candidate by week’s end, and begin to immediately deliver the drug to the states.
Over the weekend, Moncef Slaoui, the chief science adviser for the White House’s Operation Warp Speed, set out an ambitious goal, saying vaccinations could reach 24 million Americans by mid-January.
Regulators in the European Union are currently reviewing the safety and efficacy of the Pfizer vaccine and could grant authorization before the end of December.
British medical authorities defended themselves against the accusation that their regulators had acted too quickly.
Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, initially told Fox News that British regulators hadn’t acted “as carefully” as the FDA, which he called the world’s gold standard for drug review.
He quickly apologized for his remarks, saying he meant to say that British and American regulators do things differently, not better.
June Raine, chief executive of Medicines and Healthcare Products Regulatory Agency, which regulates vaccines in Britain, said she had complete confidence in the Pfizer vaccine.
“The highest standards of scrutiny, of safety and of effectiveness and quality have been met, international standards,” she said.
Raine said the immunization program beginning on Tuesday “will help us turn the corner. There’s really not one of us who hasn’t been affected by this pandemic, and our organization, like every other, has been completely focused on doing our job to be able to help defeat this terrible disease.”
One big question, though, is how many people will be willing to get a shot. More than a third of people in Britain say they are unlikely to accept a vaccine when available, according to an opinion poll published Sunday by the Observer newspaper.