Historically, animal experiments have been used to perform toxicity and safety tests during the development of novel pharmaceutical, chemical or cosmetic products. However, animal testing can be expensive, time-consuming and more information is surfacing questioning the physiological relevance and bias associated with using such models. In addition, animal advocacy organizations have fueled awareness of the ethical implications of using such models, urging the pharmaceutical industry to move toward nonanimal testing strategies.
We recently spoke with Pascal Descargues, CEO at Genoskin to learn more about the legal restrictions on animal testing and how human skin tissue can be used as an alternative. Descargues explains how Genoskin’s human skin models are created, discusses how these models can impact the drug development process and elaborates on how they are helping to develop therapeutic and vaccine strategies against COVID-19.
Laura Lansdowne (LL): What are the key legal restrictions and ethical considerations related to animal testing and how can human skin tissue models be used to alleviate these challenges? What key benefits do these skin models provide?
In the US, animal testing conducted by pharma companies is legal and required by the US Food and Drug Administration (FDA) before a new therapy can advance to human clinical trials and ultimately receive approval. However, six US states and the entire EU have banned animal testing for cosmetic use. The Environmental Protection Agency said by 2025 it will cut funding for mammalian experiments in half and that it will eliminate all mammal testing for the approval of new chemicals by 2035.
Genoskin is offering pharma and cosmetic industries a safe, accurate, sustainable and accessible standardized living human skin platform that can be used as an alternative to animal testing, or in conjunction with it. Our ex vivo (living human skin) technology is the only living human skin platform on the market. With this technology, we can test the effects of drugs in the skin (pharmacodynamics) and investigate how the skin can also affect the drug (pharmacokinetics). Our mission is to demonstrate that human skin models are more accurate, predictive and ethical compared with animal models. Pharma and cosmetics industries as well as research institutes are beginning to realize that animal testing is an insufficient predictor of drug safety and/or efficacy in humans. Additionally, animal testing is often cruel and inhumane.
Molly Campbell (MC): Can you expand on how human skin tissue models are created for use in the laboratory?
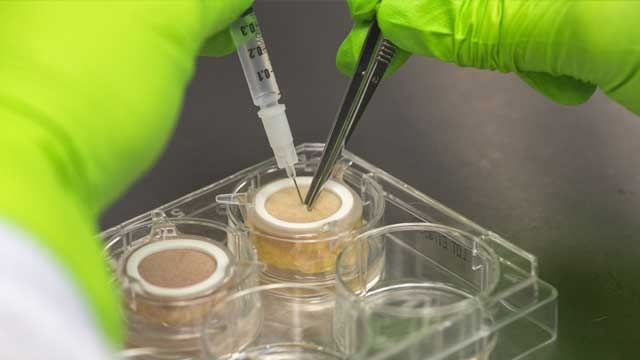
Working with donors, clinicians and hospitals, we collect human skin “leftovers” from facelifts, tummy tucks and other elective procedures. All donors express consent to donate excess tissue and then every skin sample is tested for safety before it is integrated into our testing kits. Our live human skin models hold all the characteristics and appendages of real human skin and allow companies to test products on an innovative skin model that provides adequate skin barrier function, tissue integrity, morphology and physiology. Our models represent the last stage of preclinical screening in laboratory conditions, prior to clinical evaluation. These samples can tan, heal and even grow hair. They can be used to test everything from the effects of UV light on skin, to immune responses and toxic substances.
LL: Can you elaborate on the technology used to enable the human tissues to remain alive and functional ex vivo?
Using the skin samples, we produce round skin biopsies, approximately an inch in diameter, and then put them into a proprietary nourishing gel-like matrix. Our skin model consists of normal human skin that is kept alive for seven days in multi-well testing kits, to enable much broader efficacy and toxicity studies. We distribute these living skin assays, maintained in a functional survival state, in a gel.
LL: Could you discuss some of the key applications to which these skin models can be applied?
Genoskin has published data with partner companies including GSK, Sanofi, AstraZeneca, LEO Pharma, Harvard University and many others. Our platform is being used for testing in numerous disease areas, including but not limited to; cancer, metabolic disease and neuronal disease. Pharma looks to determine how a drug that is administered through human skin, topically or subcutaneously, can induce toxicity in the skin. They also want to understand how a drug administered in the body can be changed by skin activity or inflammatory cells.
MC: How can skin models speed the process for companies developing vaccines for COVID-19? Can you provide any specific examples of currently ongoing research?
We are actively working in this field and have launched a preferred access program for researchers actively developing therapeutic and vaccine technologies for the COVID-19 pandemic.
The program covers all of Genoskin’s ex vivo assay platforms, including the innovative HypoSkin® model, the world’s first injectable real human skin assay. Vaccines are typically first tested on animals, and then once enough preclinical data is collected, the companies will develop and commence human clinical trials. We use the ex vivo platform to inject the vaccine and to test the immunological signature inducted by the vaccine to predict if a vaccine can be effective or not. This does not imply today that we can skip testing in humans – it is mandatory – but it means that you can produce additional data to support the development of the vaccine and it can help to design a robust clinical trial to ensure the best outcome. This is about de-risking. Pharma does not want to waste time or money by launching a clinical trial that is not effective or is unsafe. Our data is more accurate than that obtained by animal testing and allows pharma companies to progress to the clinical trial phase of development faster.
MC: Are there any differences in the data required by regulatory authorities for preclinical testing of a novel therapeutics using skin tissue models vs animal models?
We believe our technology is a better predictor of a drug effect in human and is more accurate and reliable than animal models. The human skin and specific gel-like matrix we designed does not contain any animal component. We have two main drivers. The first one is ethics, as there is more and more consumer and regulatory pressure to move away from animal experimentation. The second one is science. Despite the positive results that can be obtained during preclinical animal testing, more than 90% of drugs entering clinical trials fail and never receive approval. Most of them fail due to toxicity or weak efficacy that was undetected in animals. We provide a solution. We are here to help access real human live data even before a clinical trial in humans.
MC: Are there any specific challenges associated with using human skin tissue models?
Production of real human skin models is dependent on donors. Genoskin has developed a wide network of clinicians and hospitals in France and the US. We are constantly looking to expand that network to be sure to tackle any availability issues. We also work on raising patients’ awareness to gain more donors.
Pascal Descargues was speaking with Molly Campbell and Laura Elizabeth Lansdowne, Science Writers for Technology Networks.