Q: Are there any at-home tests for the coronavirus that causes COVID-19?
A: Several companies are trying to fill the need for at-home testing for the coronavirus, either through at-home tests that deliver results within hours, or kits that allow individuals to take their own samples and send them to a commercial lab for testing.
“The option of having an at-home test mailed next-day to your door gives Americans nationally a new option for how to get tested without putting others at risk of infection by driving to a lab or doctor’s office,” Paul Johnson, CEO and co-founder of telemedicine provider Lemonaid Health, said in a release. Lemonaid is partnering with Scanwell Health on an at-home coronavirus test.
However, no at-home tests or sample collection kits are currently approved by the Food and Drug Administration for the coronavirus. The FDA has barred the use of at-home sample collection for coronavirus testing, warning that “fraudulent health claims, tests, and products can pose serious health risks.”
As a result, hopes for a fast rollout of at-home tests or collection kits have slowed.
Health care startup Carbon Health had recently begun shipping $168 home sample collection kits, according to a Business Insider article. After the FDA’s announcement, Carbon Health stopped selling the kits on its website.
“We then contacted all patients who had been distributed a sample collection kit, refunded their payment, asked them to destroy the sample collection kit if they had not returned them, and offered them testing in our clinics through LabCorp or Quest Diagnostics,” Carbon Health co-founder and CEO Eren Bali said in a statement.
Health care startup Nurx has stopped taking all new requests for its coronavirus home sample collection kits, according to its website. It was unclear whether the company had begun selling and shipping them, or just taking orders for future shipping.
“The most important action we can take at this time is to support public trust in the FDA during this public health emergency,” the company said on its website. “This continues to be the priority of Nurx, and we stand ready to help expand needed and appropriate testing and will continue to explore ways to be supportive in the meantime.”
Nurx’s at-home test would allow individuals to collect a throat swab and send it to a lab. Within 48 hours of the sample’s arrival, the lab would have results, Nurx said. Health care providers would reach out to patients with follow-up advice.
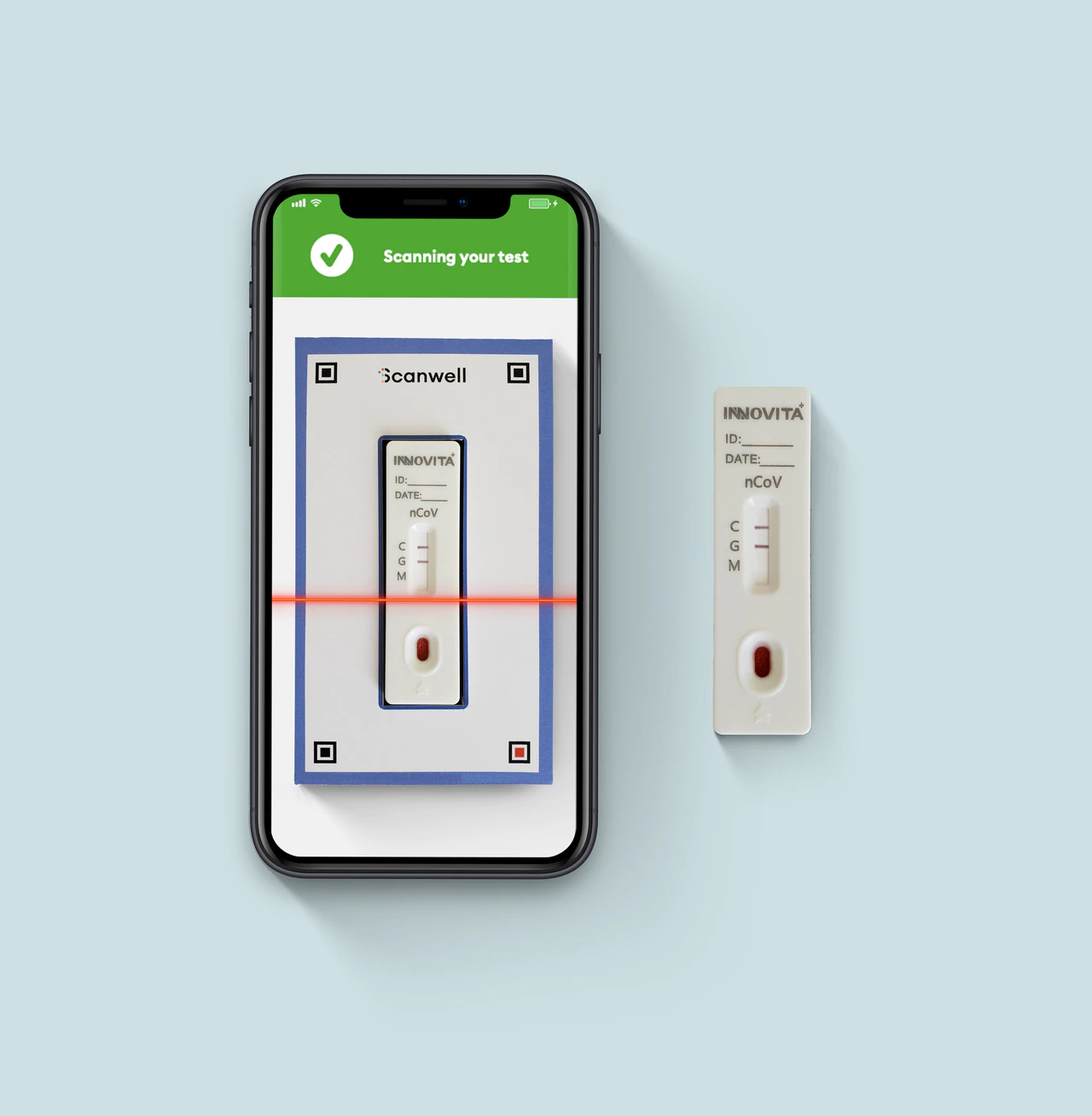
Scanwell Health may be first to market
Scanwell Health’s $70 coronavirus at-home test kit could be the first to reach the market, the company said.
“This test doesn’t require the patient to mail anything back to a lab, or drop anything off at a central collection point. The patient just stays at home, and can do everything there,” Lemonaid’s Johnson said in an email. Lemonaid is partnering with Scanwell to provide the telehealth component of Scanwell’s coronavirus test.
Patients answer questions on the Lemonaid website or via the Lemonaid app, and a Lemonaid health care professional orders the coronavirus test, Johnson said.
The patient places a single drop of blood and two drops of a separate solution, provided by Scanwell, into the test kit, Johnson explained. After about 15 minutes, the results show up as a set of lines on the testing stick. The patient takes a photo of the testing stick with the Scanwell app (using a cellphone camera) and sends the image securely to Lemonaid.
Lemonaid’s health care providers will review the image and discuss the test results with the patient via the patient’s Lemonaid app, Johnson said.
“This test doesn’t aim to identify patients who have the infection right now, but instead looks to see who’s been exposed to the virus,” Johnson said. “We can help the millions of Americans who are going to want to know whether they’ve already been exposed to the virus, so that they can make informed decisions about when to go back to work and return to normal social interactions.”
Scanwell Health never sold its coronavirus at-home kits, and hasn’t paused its work. The company’s plan from the start was to seek emergency use authorization, which is used during health emergencies, from the FDA. Three weeks after gaining FDA approval, the test would be available to the states that need it most, Johnson said.
When is the FDA expected to give approval? “We don’t know. We’re just working as hard as we can with the FDA to get them what they need,” he said. “We won’t sell the kits until we get that approval, but we don’t know when that approval is coming.”
Recent Health Matters columns by Julie Washington:
Tips for effective hand-washing during coronavirus outbreak, any time: Health Matters
Tips for finding a reliable home health aide: Health Matters
Common heart tests can tell your doctor a lot: Health Matters
What are the best ways to keep reusable bags germ-free? Health Matters
Solon patient doesn’t understand $614 nasal spray charge: Health Matters
Lyme disease vaccine discontinued, but new ways to prevent it are coming: Health Matters
Hospitals say leave meds at home; readers say otherwise
Where to get help paying your medical bill